**この商品は日本国内の法律により規制されており、日本国内への発送はできません。
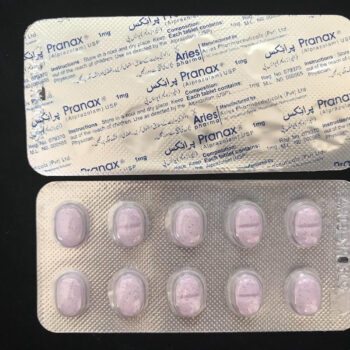
ザナックス 3mg(アルプラゾラム)USP(ザナックスジェネリック)3mg x 10錠
ザナックスとは何ですか?どのように使用されますか?
ザナックスは、不安、パニック障害、うつ病に伴う不安の症状を治療する処方薬です。ザナックスは単独で使用することも、他の薬剤と併用することもできます。
ザナックスは、抗不安薬、抗不安薬、ベンゾジアゼピンと呼ばれる薬物のクラスに属します。
ザナックスが 18 歳未満の子供に安全かつ効果的であるかどうかは不明です。
ザナックスの副作用にはどのようなものがありますか?
ザナックスは、以下のような重篤な副作用を引き起こす可能性があります。
気分の落ち込み、
自殺や自分を傷つけることの考え、
思考の加速、
エネルギーの増大、
異常な危険を冒す行動、
混乱、
興奮、
敵意、
幻覚、
制御できない筋肉の動き、
震え、
けいれん(発作)、および
心臓の鼓動や胸の動悸
。上記の症状のいずれかがある場合は、すぐに医師の診察を受けてください。
ザナックスの最も一般的な副作用は次のとおりです。
眠気、
疲労感、
ろれつが回らない、
バランス感覚や協調性の欠如、記憶障害、早朝の不安感
など、気になる副作用がある場合や副作用が治まらない場合は医師に伝えてください。
これらはザナックスの副作用のすべてではありません。詳しくは、医師または薬剤師にご相談ください。
副作用に関する医学的アドバイスについては医師にご相談ください。
説明
XANAX 錠剤には、中枢神経系活性化合物の 1,4 ベンゾジアゼピン クラスのトリアゾロ類似体であるアルプラゾラムが含まれています。
アルプラゾラムの化学名は8-クロロ-1-メチル-6-フェニル-4H-s-トリアゾロ[4,3-α][1,4]ベンゾジアゼピンです。
構造式は右に示されます。
XANAX® (アルプラゾラム)
アルプラゾラムは白色の結晶性粉末で、メタノールまたはエタノールに溶けますが、生理学的 pH では水にはほとんど溶けません。
経口投与用の XANAX 錠剤 1 錠には、アルプラゾラム 3 mg が含まれています。
用法・用量
最大限の効果を得るには、個々の患者に合わせて用量を調整してください。以下に示す通常の1日用量はほとんどの患者のニーズを満たしますが、4mg/日を超える用量が必要な患者もいます。そのような場合は、副作用を避けるため、用量を慎重に増量してください。
Anxiety Disorders And Transient Symptoms Of Anxiety
Treatment for patients with anxiety should be initiated with a dose of 0.25 to 0.5 mg given three times daily. The dose may be increased to achieve a maximum therapeutic effect, at intervals of 3 to 4 days, to a maximum daily dose of 4 mg, given in divided doses. The lowest possible effective dose should be employed and the need for continued treatment reassessed frequently. The risk of dependence may increase with dose and duration of treatment.
In all patients, dosage should be reduced gradually when discontinuing therapy or when decreasing the daily dosage. Although there are no systematically collected data to support a specific discontinuation schedule, it is suggested that the daily dosage be decreased by no more than 0.5 mg every 3 days. Some patients may require an even slower dosage reduction.
Panic Disorder
The successful treatment of many panic disorder patients has required the use of XANAX at doses greater than 4 mg daily. In controlled trials conducted to establish the efficacy of XANAX in panic disorder, doses in the range of 1 to 10 mg daily were used. The mean dosage employed was approximately 5 to 6 mg daily. Among the approximately 1700 patients participating in the panic disorder development program, about 300 received XANAX in dosages of greater than 7 mg/day, including approximately 100 patients who received maximum dosages of greater than 9 mg/day. Occasional patients required as much as 10 mg a day to achieve a successful response.
Dose Titration
Treatment may be initiated with a dose of 0.5 mg three times daily. Depending on the response, the dose may be increased at intervals of 3 to 4 days in increments of no more than 1 mg per day. Slower titration to the dose levels greater than 4 mg/day may be advisable to allow full expression of the pharmacodynamic effect of XANAX. To lessen the possibility of interdose symptoms, the times of administration should be distributed as evenly as possible throughout the waking hours, that is, on a three or four times per day schedule.
Generally, therapy should be initiated at a low dose to minimize the risk of adverse responses in patients especially sensitive to the drug. Dose should be advanced until an acceptable therapeutic response (ie, a substantial reduction in or total elimination of panic attacks) is achieved, intolerance occurs, or the maximum recommended dose is attained.
用量維持
1日4mgを超える用量を投与されている患者には、定期的な再評価と減量の検討が推奨されます。市販後対照試験において、1日4mgを超える用量のザナックスを3ヶ月間投与された患者は、臨床的ベネフィットの明らかな低下なく、総維持用量の50%まで漸減することができました。離脱の危険性があるため、治療の急激な中止は避けてください。(「警告」、「使用上の注意」、「薬物乱用および依存」の項を参照)
ザナックスに反応を示すパニック障害患者に必要な治療期間は不明です。発作が長期間消失した後、慎重な監督下で徐々に中止を試みることは可能ですが、症状の再発や離脱現象の発現なしに中止を達成することはしばしば困難であるというエビデンスがあります。
投与量の減少
離脱の危険があるため、治療の突然の中止は避けるべきです。
すべての患者において、治療を中止する場合、または1日用量を減らす場合は、徐々に減量してください。特定の中止スケジュールを裏付ける体系的なデータは収集されていませんが、1日用量を3日ごとに0.5mgを超えて減量しないことが推奨されます。患者によっては、さらに緩やかな減量が必要となる場合があります。
いずれの場合も、用量の減量は厳重な監視下で段階的に行う必要があります。重大な離脱症状が現れた場合は、以前の投与スケジュールを再開し、症状が安定した後にのみ、より緩やかな投与中止スケジュールを試みる必要があります。パニック障害患者を対象にした市販後投与中止対照試験において、この推奨漸減スケジュールとより緩やかな漸減スケジュールを比較したところ、ゼロ用量まで漸減した患者の割合に群間差は認められませんでしたが、緩やかな漸減スケジュールでは離脱症候群に伴う症状の軽減が認められました。より緩やかな投与中止が有効な患者もいることを念頭に、3日ごとに0.5 mgを超えて投与量を減量しないことが推奨されます。患者によっては、すべての投与中止レジメンに抵抗性を示す場合があります。