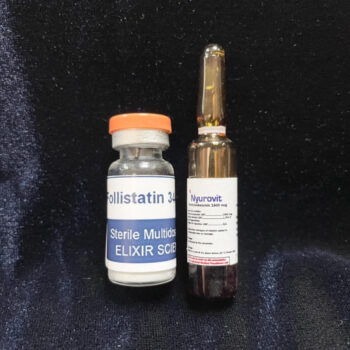
In stock
Ace031 5mg + water ampoule for injection + Bonus, expired vitamin B12 (1,500mcg/2ml) ampoule
$224
** Customers to Japan **
If the shipment is found at customs as an injection, you will be required to show proof of drug supervision from 2020.
If you cannot prepare the proof of drug supervision, please follow the procedure to return it to the sender.
We will notify you when the item arrives at our company, so we will re-ship it after paying the shipping fee again.
** Please understand that this product cannot be refunded after it has been sold.
Ace031 5mg +water ampoule for injection
+ Bonus, expired vitamin B12 (1,500mcg/2ml) ampoule
The expiration date for vitamin B12 has passed.
If you experience symptoms such as pain or swelling after injecting myostatin inhibitors, dissolve it in vitamin B12 injection solution and take it to relieve the symptoms. Since the expiration date for vitamin B12 has passed, we are including it as a bonus, so please use it at your own risk.
ACE-031 is a soluble form of activin receptor type IIB (ActRIIB). ACE-031 promotes muscle growth by binding to myostatin and other negative regulators of muscle mass.
The recommended intake of ACE031 is 0.02-3 mg / kg of body weight.
So if you weigh close to 100 kg, you need to take at least 2 vials (2 mg) at a time.
Generally, one vial is taken subcutaneously or intramuscularly per week.
Of course, if there is no cost problem, a higher intake is more effective.
In addition, it is said that 8-10 weeks is the best cycle.
Also, by using a myostatin inhibitor, you will be able to handle a heavier weight than you have handled so far, which may hurt your tendons and joints, so many people say ACE031.
By stacking and using CJC1295DAC and CJC1295DAC, it is often the case that a cycle is formed while recovering joints and tendons.
CE-031 is a myostatin inhibitor (GDF-8 – a substance which can decelerate and restrain muscle growth). By blocking myostatin, ACE-031 supports faster, greater and more efficient muscle gain.
Moystatin (GDF-8 = Growth differentiation factor 8) is a protein that in humans is encoded by the MSTN gene. Moystatin (GDF-8) belongs to the GDF proteins family that inhibits muscle growth. The moystatin protein is produced primarily in the skeletal muscle cells, circulates in the blood and acts on muscle tissues – it slows down the stem cells differentiation (and inhibits new muscle mass production). However, an exact mechanism of action remains unknown.
Myostatin and its related gene were discovered in 1997. Genetics doctors Dr. Se-Jin Lee and Alexandra McPherron found out that the cause of abnormal extra-huge muscles of the two breeds of cattle, Belgian Blue and Piedmontese, had the damaged myostatin genes (this muscle feature of these cattle breeds is known as “double muscling”).
They unnaturally produced a strain of mutant mice that lacked myostatin. As a result, these mutant mice had approximately twice as much musculature as normal mice. These mice were subsequently named as “mighty mice”. Mutation of the moystatin gene results in an inability of the body to produce its own myostatin and then myostatin cannot inhibit the muscle growth.
Afterwards, these mutations started to be used for breeding new muscular cattle breeds. Genetic mutations of the myostatic gene were observed also in a breed of racing dogs closely related to greyhounds (Whippet). Individuals with the myostatin gene mutation were apparently more capable runners and they featured different overall appearance. It is not disputed that animals lacking myostatin or animals treated with doping substances that block the myostatin activity (such as, e.g. Follistatin) have significantly larger muscles. Even a 20% reduction in moystatin levels has a great effect on the muscle development.
In 2004, a German boy was diagnosed with a mutation in both copies of the myostatin-producing gene, making him considerably stronger than his peers. As the boy is growing, he is becoming stronger than his peers. The lack of myostatine has not yet reflected on his overall health condition. Doctors will have to continue to observe him closely just in case, he could develop hypertrophy of the vital organs (e.g. heart hypertrophy). His mother, a former female sprinter, has a mutation only in one copy of the gene. People with a mutation in both copies of the MSTN gene in each cell (homozygotes) have significantly increased muscle mass and strength than common people; therefore, they appear stronger than the standard level. People with a mutation in one copy of the MSTN gene in each cell (heterozygotes) also have increased muscle bulk, but to a lesser degree.
In 2013, Acceleron Pharma published results of a human study, in which 48 healthy women aged from 45-75 were given a single injection containing 0.02, 0.05, 0.1, 0.3, 1 or 3 mg ACE-031 per kg bodyweight. The single injection did lead to muscle growth. The 3 mg/kg dose led to an increase in muscle volume by 5 percent, it boosted lean body mass by 3 percent and additionally, it seemed to also reduce fat mass. The injection reduces also the leptin concentration. This would suggest that ACE-031 decreases actively the fat mass level. ACE-031 remained in their body through several weeks after administration and the half-life was estimated to 10-15 days.
The myostatin inhibition leads to muscular hyperplasia (growth of new muscle fibres) and significantly larger hypertrophy (growth and volume increase of already existing muscle fibres). From this point of view, those products truly have a great future potential for sportsmen who aim at the increase of muscle growth.
Supposedly, one of the best bodybuilders ever – the phenomenal Flex Wheeler – has a lack of myostatin, as well.
THE EFFECTS OF USING ACE-031:
ACE-031 は筋肉量と筋力を大幅に増加させ、オキシメトロンなどの最も効率的なハードコア ボリューム ステロイドにさえ匹敵すると言われています。
ACE-031を摂取すると、筋肉の硬度が急速に、ほぼ即座に増加します。
ACE-031は脂肪燃焼を促進する
用量:
ACE-031 を 1 mg という少量でも、大きな効果が得られます。3 ~ 4 週間にわたって週 1 回 1 mg の ACE-031 を摂取したスポーツ選手は、筋肉の硬度がほぼ即座に高まり、筋肉量と筋力が急速に増加したと報告しています。これは、オキシメトロンなどの最も効率的なハードコア ボリュームのアナボリック アンドロゲン ステロイドをも上回るレベルです。
別の投与オプションとしては、1 日あたり 200 mcg の ACE-031 の投与から開始し、毎週 100 mcg ずつ投与量を増やすというものがあります。治療サイクルは 3 ~ 4 週間続きます。
ACE-031 のサイクルが完了したら、2 週間の休止期間を設けることをお勧めします。
この製品の純度は98.42%です。
これは、COA および HPLC テスト レポートによって検証された最高水準の処方です。